

Therefore the idea was tested with enone 3, which was prepared from commercially available oxazole ester 1 in two steps.

Gratifyingly, treatment of 3 with aqueous NaOH afforded pyrrole 4 in 82% yield. Later, it was found that compound 3 could be prepared by aldol reaction followed by elimination of the mesylate (scheme below). It was necessary that the mesylate formation from aldol 6 was complete before the addition of NaOH for the synthesis of pyrrole 4 in one pot to be effective. In this manner, the elimination of the mesylate with NaOH to form the enone occurred quickly compared to using Et3N.

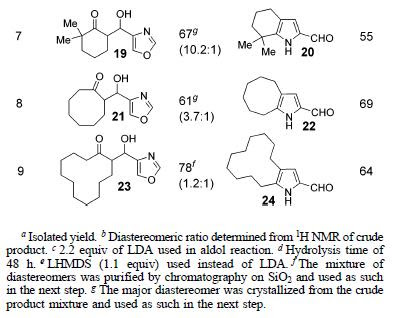
After the optimal conditions for the 2-formylpyrrole synthesis were established, the scope of the reaction was explored and the results are summarized below.


Extension of the methodology to beta-(4-thiazolyl)-enone was explored. It was planned that if the reaction pattern is the same as the oxazole, then 2-thioformylpyrrole could be prepared. However, it was found that the reaction did not proceed when 26 was subjected to the same reaction conditions (recovered SM).

It was suspected that thiazolium salt may be necessary for the hydrolysis with NaOH to occur. Therefore, the thiazole was alkylated with benzyl bromide before being subjected to the NaOH hydrolysis. However, only complex mixture of products was obtained. It was suspected that the intermediate A was not stable under the reaction conditions. Thus, MeI was added to trap A as sulfide B. Under the reaction conditions, the intermediate B underwent an exchange with OH to give intermediate C which eventually underwent the same reaction pathway to give pyrrole 28 in 64%.
Although the reaction of thiazole 26 failed to give the anticipated 2-thioformylpyrrole, it provided an effective route to N-benzyl-2-formylpyrrole.
No comments:
Post a Comment