
I guess the major problem in working with cocaines is how to access to the substance since it is illegal to possess. This is what the authors had to say:
"Although not commercially available,confiscated grade cocaine can be obtained from the National Institute on Drug Abuse with appropriate DEA licensing in sufficient quantities to provide useful amounts of chiral building blocks."
So it is possible to get some. This is good information. What embedded in cocaine is the cis-dialkyl substituent on the pyrrolidine ring as in 2 through a series of chemical degradation. This cis-relationship was also found in some indolizidine-type natural products, as in 1.
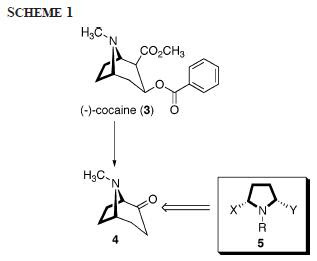
Starting with (+)-2-tropinone 4, demethylation followed by Cbz installation afforded 6. The process was conducted to decrease the basicity of nitrogen and to protect nitrogen from being oxizided in the subsequent step. Usual chemical operations ensued to provide protected pyrrolidine 8 in good overall yield (Scheme 2). It was found that compound 8 existed as a mixture of rotomers which made it difficult to be properly characterized.

Onto the synthesis of (-)-monomorine, the synthetic sequence is illustrated in the scheme below.

The usual synthetic operations led from compound 8 to 11. The double bonds were then hedrogenated and at the same time with the deprotection of N-Cbz group. The free nitrogen then cyclized with the ketone carbonyl to form the intermediate imine which was hydrogenated under the reaction conditions to give the desired product 12 in 87% as a single enantiomer. This was in agreement with previous report (Conchon, E.; Gelas-Mialhe, Y.; Remuson, R. Tetrahedron: Asymmetry 2006, 17, 1253.) that in hydrogenation of imine double bond, hydrogen is delivered from the face syn to the hyfrogen at the 8a position on the ring.
Thus the paper demonstrated the successful and convenient way of generating cis-2,5-dialkylpyrrolidine from cocaine. The article also illustrated the utility of this useful chiral building block in a successful total synthesis of (-)-monomorine.

1 comment:
Well said.
Post a Comment