I have only skimmed through the paper. But I think this is a neat method demonstrating the use of N-heterocyclic carbenes or NHCs in a basic organic reaction for the formation carbocycles (1 to 2).

The mechanism of this process is proposed as followed.

The reaction conditions were screened with four different NHC compounds. The screening results are summarized below.
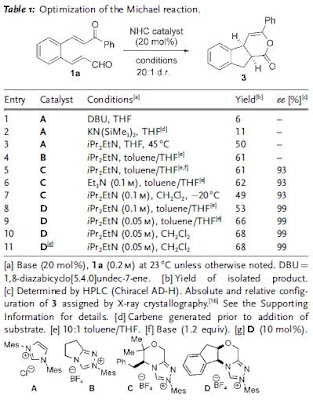
Triazolium salt D was found to be the most effective in term of both yield and ee of product 3. The scope of the reaction was explored and the results are in Table 2.


- The use of methanol to quench the reaction avoids the propensity for several of the bicyclic products to undergo hydrolysis when purified on silica gel.
- The optimized reaction conditions allowed both electron-withdrawing and -donating groups on the enone (entries 1–3).
- Additionally, electron-withdrawing and -donating substituents could be placed on the aromatic tether (entries 6 and 7).
- The alpha,beta-unsaturated methyl ketone 1d provided a moderate yield of the cyclopentane product with excellent enantioselectivity (entry 4).
- The bisaldehyde 1e underwent an interesting desymmetrization reaction in which one aldehyde became the nucleophile when exposed to an NHC while the other unsaturated moiety became the conjugate acceptor (entry 5).
- The cyclization of the aliphatic substrate 13 (entry 8) proceeded in good yield after ten hours with a catalyst loading of 20 mol%.
- When the tether length was increased to access six-membered rings, cyclohexene products were afforded but with reduced enantioselectivity and yield (62%ee, 52%; entry 9). Interestingly, product 16 did not open after the addition of methanol, unlike the cyclopentane compound.
The method also allows access to amide-substituted carbocycles when amines, instead of metahnol, were used to open the ring.
Nice chemistry which seems to have practical uses. NHCs have become an efficient and popular tool in organic synthesis. And chemists have started to explore the use of this class of compounds in reactions, other than only as ligands for Grubbs or Hoveyday-Grubbs metathesis catalysts. In fact, recently a book on NHCs in organic synthesis has been published by Wiley.



 In addition to DBU and Hunig's base, alkaloid such as cinchonine was also found to be an effective catalyst as demonstrated in the example below.
In addition to DBU and Hunig's base, alkaloid such as cinchonine was also found to be an effective catalyst as demonstrated in the example below.












 The mechanism was proposed to involve addition of NHC to aldehyde to form the extended Breslow intermediate I, followed by reaction of this intemediate with azomethine imine to give intermediate II. This intermediate then tautomerized to give III which intramolecularly acylated to give product 3.
The mechanism was proposed to involve addition of NHC to aldehyde to form the extended Breslow intermediate I, followed by reaction of this intemediate with azomethine imine to give intermediate II. This intermediate then tautomerized to give III which intramolecularly acylated to give product 3.

 The reaction tolerated various R groups on aldehyde including aliphatic (entry 6) and extended olefin (entry 7). However, when R group was eletron-withdrawing group-substituted phenyl ring, the reaction did not yield any product (entry 8).
The reaction tolerated various R groups on aldehyde including aliphatic (entry 6) and extended olefin (entry 7). However, when R group was eletron-withdrawing group-substituted phenyl ring, the reaction did not yield any product (entry 8). Further transformations of pyridazinone 4 were found to be facile both with MeOH and BnNH2 to give the corresponding ester 19 and amide 20 in almost quantitative yields.
Further transformations of pyridazinone 4 were found to be facile both with MeOH and BnNH2 to give the corresponding ester 19 and amide 20 in almost quantitative yields.









