Susumu Saito, Tetsu Tsubogo, and Sh Kobayashi*
Graduate School of Pharmaceutical Sciences, The University of Tokyo, The HFRE Division, ERATO, Japan Science Technology Agency (JST), Hongo, Bunkyo-ku, Tokyo 113-0033, Japan
This paper presented a novel metal catalyst system in conducting a 1,4-addition of glycine derivative to alpha,beta-unsaturated carboxylic acid derivative to give glutamic acid derivative as a product. The novel catalyst being used in the reaction was Ca2+ salt in conjunction with chiral ligand.
Initially, reaction was screened to find the most optimal conditions. This included the screening of metal salts and ligands and the results are summarized as followed.

In the end, ligand 4 was found to be the most optimal ligand along with Ca(Oi-Pr)2 salt. Upon identifying the best conditions, the scope of the reaction was investigated. The reactions with various substrates were mostly found to proceed in great yields and stereoselectivity as seen in Table 2.

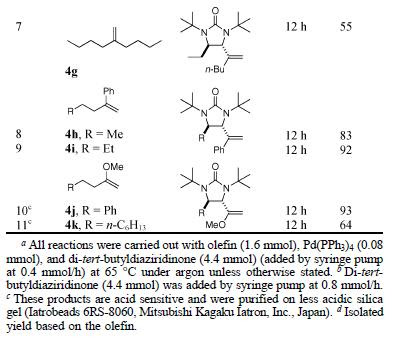
Next, the reaction was screened with crotyl carboxylic acid derivatives. It was surprisingly found that instead of the 1,4-addition addition product, pyrrolidine derivative 7 was obtained in a formal [3+2] cycloaddition reaction. Several substrates were then explored and the results are summarized below. In most cases, the reactions proceeded in excellent yields and diastereo- and enantioselectivities.

The catalytic cycle was proposed to be as followed.

In a regular 1,4-addition, intermediate 10 was protonated to give product 6. But when the amide derivative was used as the Michael acceptor, the intermediate 10 became more reactive and intramolecular cyclization occurred to give pyrrolidine 7. However, from both Tables 1 and 2, this distinction was not very clear as the only difference in conditions between the two reactions seemed to be the reaction time. Therefore (in my opinion), it is more likely that the reaction intramolecularly cyclized faster (in 3 h). But when the reaction was allowed to react longer (12 h), retrocyclization occurred and the initial pyrrolidine derivative product (formal [3+2]) was transformed to the glutamic derivative product (1,4-addition).
Ligand 4 was suspected to be an anionic ligand as when structurally-related ligand 8 (which was expected to form a neutral complex) was used instead of 4 in a reaction between methyl acrylate and 1a, the reaction proceeded in only 31% and provided the product in racemic form.



























 The reaction was also shown to be stereospecific, that is the stereochemistry in the starting material was effectively transferred to the product as shown in the scheme where stereochemistry of carbon bearing the phenyl group in 1a was retained in product 5a.
The reaction was also shown to be stereospecific, that is the stereochemistry in the starting material was effectively transferred to the product as shown in the scheme where stereochemistry of carbon bearing the phenyl group in 1a was retained in product 5a.















 It should be noted in entry 5 that when ortho-substituted aromatic group of imine 21 was used, the reaction occurred diastereoselectively to give a 4:1 atropisomeric mixture.
It should be noted in entry 5 that when ortho-substituted aromatic group of imine 21 was used, the reaction occurred diastereoselectively to give a 4:1 atropisomeric mixture.



