From Prof. Dirk Trauner's group at UC Berkeley
The synthesis is asymmetric, concise, convergent, and very short with 6 total linear steps from know materials. 

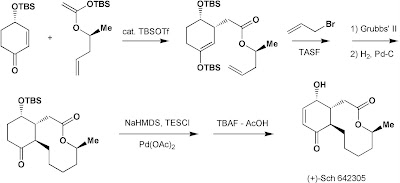
Steps employed in stitching these three pieces together include the Mukaiyama conjugate addition of the silyl ketene acetal catalyzed by TBSOTf which preferentially gave rise to syn product (3.7:1) due to the "Cieplak" effect. Then TASF- (tris(dimethylamino) sulfonium difluorotrimethylsilicate) mediated allylation with allylbromide led to the precursor for RCM. The metathesis was carried out using Grubbs' 2nd generation catalyst to give the cis-alkene in the macrocyclic lactone ring. Hydrogenation ensued in excellent yield, followed by the Saegusa-Ito unsaturation through TES enol ether of the ketone carbonyl. The final step involved a carefully-bufferred deprotection of the OTBS with TBAF.
 A short and nice synthesis and a good read.
A short and nice synthesis and a good read.
No comments:
Post a Comment