Seth B. Herzon and John F. Hartwig*
Department of Chemistry, University of Illinois, 600 South Mathews Avenue, Urbana, Illinois 61801
A new reaction which will probably become a name reaction: the Hartwig Hydroaminoalkylation.
Department of Chemistry, University of Illinois, 600 South Mathews Avenue, Urbana, Illinois 61801
A new reaction which will probably become a name reaction: the Hartwig Hydroaminoalkylation.

This was based on the work done over two decades by Maspero and Nugent, according the scheme below.

The reaction essentially added aminoalkyl group across the double bond of an olefin. The aminoalkyl group is part of the N-methylaniline and. The catalyst was screened as presented in Table 1 and Ta[N(CH3)2]5 was found to be most optimal.
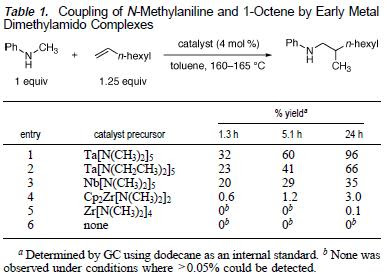
The reaction was found to work well with a variety of terminal olefins, which in most cases, providing methyl-branched products in good yields (except entry 2 below where a mixture of products was obtained).

A variety of arylamine partners could also be utilized as shown in Table 3.

The mechanism of the reaction is largely under investigation. Nonetheless, this is a neat reaction.
No comments:
Post a Comment