From Prof. Jin Kun Cha's group at Wayne State University, Detroit, MI
An ACIEE EarlyView paper from Prof. Cha involving with diastereo- and enantioselective construction of 2,4,6-trisubstituted tetrahydropyran (THP). The methodology described is based on trapping of oxocarbenium intermediate by proximal R3SiOTf-promoted cyclopropanol ring openning. Scheme shows the general reaction method and partners involved.

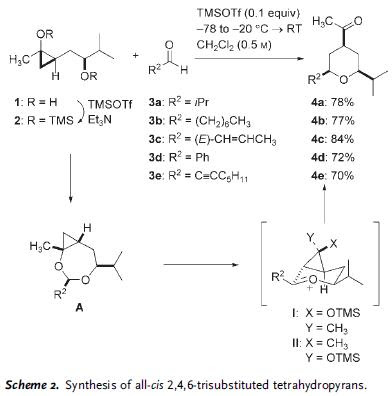
The reaction proceeded through cyclic acetal intermediate A, followed by Lewis acid-promoted cyclopropanol ring openning concurrently with trapping of the nascent oxocarbenium species (I and II). It was later found that step-wise operation to form cyclic acetal, followed by oxocarbenium formation was not necessary. The entire sequence could be performed in one step by treatment of substrate 1 with 2.5 equiv of TMSOTf.
In preparation toward natural product synthesis, the coupling of two related segments 6 and 7 was performed. Under reaction conditions, however, the desired product 9 was obtained along with undesired elimination product 10.
In an effort to optimize this step, cyclic acetal 8 was pre-formed and then subjected to silyl triflates. The acceptable result was obtained with TIPSOTf. During this optimization, it was discovered that this required process was actually promoted by triflic acid, generated from silyl triflate as evidenced from the fact that the reaction could be shut down in presence of base.
By starting from chiral alcohol 11, both chiral 6 and 12 could be prepared. Coupling of this two fragments gave rise to enantio-pure all cis THP product, such as 13 (see below). Further manipulations afforded the bis-THP system 15 in good enantioselectivity with THP rings in all cis arrangement.
In another illustration of the utility of this method is shown in the rapid synthesis of (+)-18 starting with all enantio-pure starting materials.
When the relationship of cyclopropane and the OR in the tethered chain were trans as in 19, reaction with 3b afforded product 21 selectively over 22 (10:1) via the intermediacy of III as shown below.
This method has shown a lot of potential to be applied to syntheses of natural products, especially with the readily available enantio-pure starting materials, enantio- and diastero-pure THP products could be formed in good to excellent yields and selectivities due to the reaction's high stereospecificity.




1 comment:
Thanks for this. I really like what you've posted here and wish you the best of luck with this blog and thanks for sharing. Triflic
Post a Comment