Link: ACIEE EarlyView
Takahiro Nishimura,*, Taisuke Katoh, Tamio Hayashi*
Department of Chemistry, Graduate School of Science, Sakyo, Kyoto 606-8502, Japan
Takahiro Nishimura,*, Taisuke Katoh, Tamio Hayashi*
Department of Chemistry, Graduate School of Science, Sakyo, Kyoto 606-8502, Japan
The method shown in the paper demonstrated the use of Rh to transfer aryl group from tertiray substituted methanol to alpha,beta-ketones and ester.
The Rh-Aryl bond is formed via beta-elimination of the tertiary alcohol. This transformation also paralleled to methods known previously using other metals.

In effecting this transformation, alcohol 1 was used as the source of aryl group. Other aryl sources were also studied (4m-8m for Ph, Scheme 3) but 1 was found to give the best result, yielding the desired 1,4-adduct 9 and ketone 2 as a byproduct.


The results using 1m-u are summarized in Table 1.

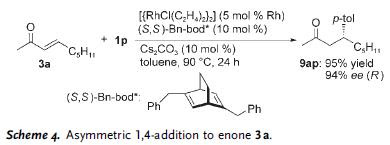
Additionally, the reaction could be conducted with stereoselectivity when (S,S)-Bn-bod* was used in place of cod on the Rh catalyst. This is demonstrated in the reaction between 3a and 1p in Scheme 4.
The mechanism was proposed as shown in Scheme 6.


1 comment:
Undeniably imagine that that you stated. Your favorite justification appeared to be at the web the easiest factor to understand of.
I say to you, I definitely get annoyed even as folks think about
concerns that they just don't know about. You managed to hit the nail upon the highest as neatly as outlined out the whole thing with no need side-effects , other people could take a signal. Will probably be back to get more. Thank you
Review my homepage - toddlerhitting.org
Post a Comment