Hye-Young Jang, Jun-Bae Hong, and David W. C. MacMillan*
Merck Center for Catalysis at Princeton University, Princeton, New Jersey 08544, and Division of Chemistry and Chemical Engineering, California Institute of Technology, Pasadena, California 91125
In continuing their investigation in the newly discovered mode of organocatalysis - the singly occupied molecular orbital, or SOMO activation - MacMillan group presented a new reaction. In the original contribution, alpha-allylation of aldehyde using allylsilane was recently reported.


This time alpha-enolation of aldehyde and enolsilane using the same catalyst system was reported for the first time. The mode of activation is through a single-electron activation with CAN, as shown in the scheme below, to give the corresponding electron-deficient radical cation.
Based on a DFT calculation, the enolsilane or the "SOMOphile" would approach the "SOMO-catalyst" on the si-face to avoid the bulky t-Bu group of 1, establishing the enantioselectivity of the reaction.

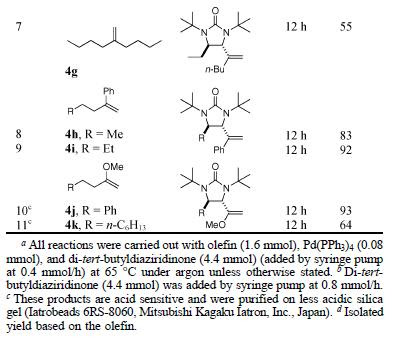
Thus, using enolsilane 3 as a somophile, reactions with various aldehydes proceeded in good yields and ees as shown in Table 1.

In addition, several somophiles were used to react with octanal to give the expected products in good to excellent yields and excellent ees (Table 2).


In addition, the reaction was found to be very mild and chemoselective as illustrated in Eqs 5 and 6. In a normally difficult-to-control intramolecular radical cyclization of 4, when reaction was conducted in the presence of enolsilane, the corresponding alpha-enolation product was obtained selectively in excellent yield and ee.

SOMO-activation has become more important in the field of organocatalysis. One could expect to see much more of this in the near future.






 The reaction was found to be stereosepcific, that is the configuration of the benzylic position in the SM is retained in the product. The reaction was found to be widely applicable regardless of electronic character of the aryl ring being transferred.
The reaction was found to be stereosepcific, that is the configuration of the benzylic position in the SM is retained in the product. The reaction was found to be widely applicable regardless of electronic character of the aryl ring being transferred.













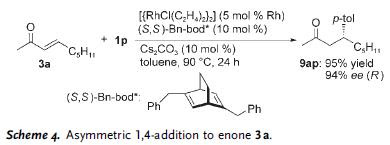



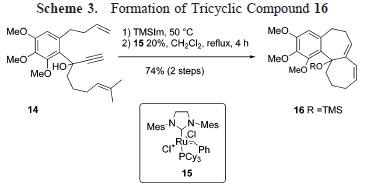












 In the subsequent scheme, further utilities of the reaction is illustrated, using Hunig's base as catalyst. In some of these reactions, cyclic acetal was obtained along with the expected aldol adduct.
In the subsequent scheme, further utilities of the reaction is illustrated, using Hunig's base as catalyst. In some of these reactions, cyclic acetal was obtained along with the expected aldol adduct. As is readily seen, the reaction predominantly afforded syn-aldol product. The selectivity was probably stemed from the effect of hydrogen-bonding - a welcome complement to the previously known anti-selectivity.
As is readily seen, the reaction predominantly afforded syn-aldol product. The selectivity was probably stemed from the effect of hydrogen-bonding - a welcome complement to the previously known anti-selectivity. In addition to DBU and Hunig's base, alkaloid such as cinchonine was also found to be an effective catalyst as demonstrated in the example below.
In addition to DBU and Hunig's base, alkaloid such as cinchonine was also found to be an effective catalyst as demonstrated in the example below.







